Understanding Types of Studies: Exploring Clinical Trials and Real-World Evidence
Understanding Types of Studies: Exploring Clinical Trials and Real-World Evidence

When it comes to a cancer diagnosis, many people look for multiple sources of information to try to learn as much as they can to get the “full picture.” The same can be true when it comes to understanding treatments.
Medicines can be studied in multiple ways. Talking with your doctor about the results of different types of studies may help you feel more confident when making decisions about your treatment. It also gives you the opportunity to talk about what matters most to you during treatment.
This article will tell you about 2 different types of studies you may learn about or come across, how they work, and how the information can be useful to you.
Your treatment journey
It began as soon as you were diagnosed with cancer—your journey on a road filled with uncertainty about what comes next. It has likely left you feeling overwhelmed.
One way you may start to feel more in control during your journey is to talk to your doctor and learn about your treatment options. When you decide together on the option that is best for you, it is called shared decision-making. Shared decision-making happens when you share your treatment goals with your care team, play an active role in conversations about how to approach your treatment, and then decide on a treatment together.
How medicines are studied
There are a number of treatment options for most types of cancer. Knowing how these treatments were studied may be helpful when you work with your doctor to pick one that may best meet your needs. Two of the different types of research studies that can offer useful information about your options are clinical trials and real-world studies.
CLINICAL TRIALS
A clinical trial is a type of research study that can be used to help us understand how a medicine works in people. Results from clinical trials are an important type of information that the FDA uses to determine if a new drug or treatment is safe and effective. In the case of a new medicine, the FDA decides whether to approve a new treatment or a new use of an approved medicine.
Clinical trials are set up, or “designed,” in different ways. For example, one clinical trial may be designed to include 1 treatment group, where all of the participants receive the same treatment. Other designs can have more than 1 treatment group, where the participants can be split among the groups and receive different treatments. "Arms" is another name used for the treatment groups that are part of clinical trials.
They also have strict rules, or “criteria,” that help researchers determine whether what was learned in the study was a result of the treatment being given or from some other factor. These criteria can include things like patient age, gender, if they have received prior treatments, and other factors. Patients who fit within the criteria may be eligible to participate in the study.
There are several types of clinical trials. One common type is the randomized controlled trial (RCT). When it comes to studying a new or existing treatment, RCTs are thought to be the gold standard.
Once eligible patients agree to participate in an RCT, they are “randomized,” meaning they are randomly assigned to different treatment groups. This is like flipping a coin to decide who goes into which group.
A study can have several different groups but for now, let’s use the example of a study with 2 groups. The image below shows who might be eligible for a clinical trial and how they are split into different treatment groups.
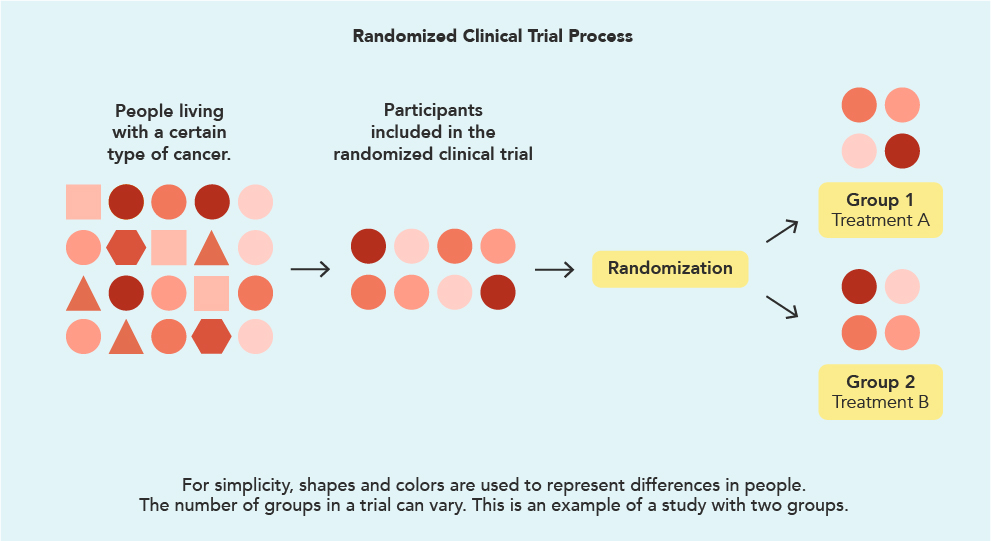
There are several ways to study a treatment and to understand the patients’ experiences with it. In the example above, the group of eligible participants who have agreed to enter the study have been randomly assigned into one treatment group or the other. The first group (Group 1) receives the treatment being studied. The second group (Group 2) receives an established treatment, known as the standard of care (this may be a routine treatment or observation and monitoring). The group that does not receive the treatment being studied is called the “control group.”
How are RCTs used?

Most often, RCTs are carried out before a new treatment is approved and made available to doctors to prescribe to their patients. They can help us understand the potential benefits and risks of a treatment. The FDA will carefully study the results of the trial and decide if the treatment is safe and effective for use in the United States for a specific condition in a specific group of patients.
REAL-WORLD STUDIES
What are real-world studies?
Once a medicine is approved for use by the FDA and more people are taking it, information about its use in actual medical practice—in the “real world”—can be gathered and studied to learn more about patients’ experiences with the treatment. This can include how well the treatment works, or even what patients say about how they feel while on treatment. This information is called “real-world evidence” (RWE).
How is RWE different from RCTs?
RWE comes from real-world studies done outside of clinical trials. RWE is not meant to replace RCTs. It is meant to provide additional information on the treatment. RCTs evaluate how well a treatment performs and how safe it is in a controlled setting. RWE reports on how that treatment performs in the “real world.”
Compared to an RCT, which is often designed to include specific types of patients, patients in a real-world study may have varying traits that were not included in the clinical trials. For example, patients with high blood pressure that is not well-controlled may not be allowed into a clinical trial. But they may be included in a real-world study.
The image below shows who might be eligible for a real-world study and how they are split into different treatment groups. Some limitations of RWE could include missing information or bias, like a physician choosing the treatment the patient received instead of the treatment being determined through randomization.
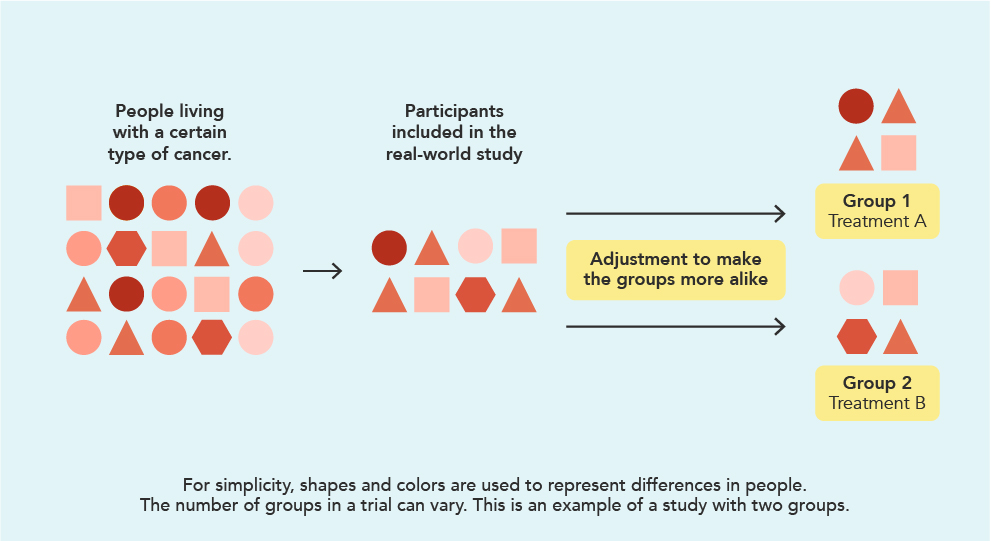
Where does RWE come from?
The RWE that comes from real-world studies is gathered from sources such as medical records, insurance claims, certain mobile apps that capture medical information, or other places where similar information can be found. These sources can provide meaningful information about how a medicine is used in everyday practice. But they do have drawbacks, like missing or incorrect information.
It's important to note that in real-world studies, the identity of any patient cannot be determined. This is because the personal information, such as the patient’s name, is removed.
Where can I find information on randomized clinical trials and real-world evidence?
RCTs have been conducted for many years. Talking to your doctor is always a great place to start, and information can usually also be found via an online search using the name of the treatment.
If you are interested in learning RWE for a specific medicine, talk to your doctor. Your doctor or another member of your healthcare team can find out if RWE exists for approved treatments. They can do this in multiple ways, including by reading medical articles or by reaching out to the manufacturer of the medicine.
Real-world studies are becoming more common, but are still relatively new. You should not be concerned if this information is not available for a treatment you are taking or considering taking.
Together, RCTs and RWE provide information that can support shared decision-making.
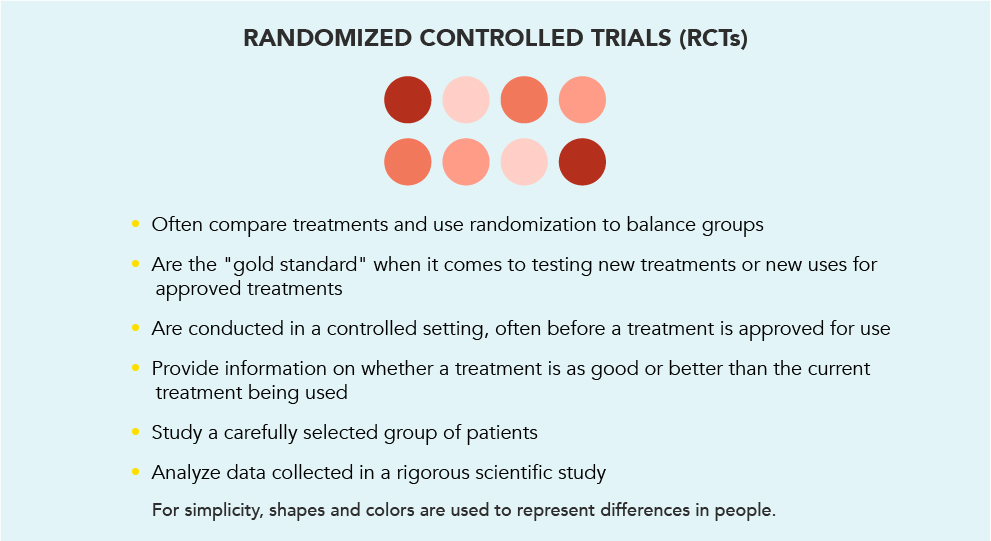
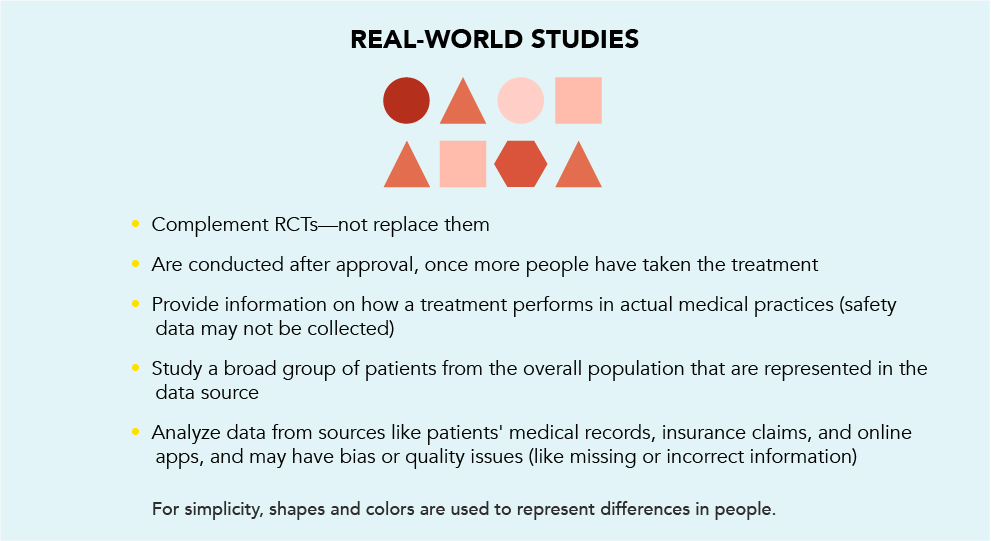
While RCTs are the gold standard for learning about how a medicine works, and if it is safe and effective for use, RWE provides another layer of information to help you make the decision that's right for you. By talking to your doctor about RWE, you may discover information about how patients who can seem more like you experienced the treatment.
GETTING STARTED
A little knowledge can go a long way
For many patients, whether they are newly diagnosed or have been living with cancer, talking to their doctor about treatment can feel like a brand-new experience. This is normal. But you may find that asking questions and reading up on your type of cancer may increase your confidence, help you make more informed decisions, and learn to be your own advocate.

How can I start a conversation with my doctor?
You are your own best advocate, so be sure to tell your doctor that you have topics you’d like to discuss. A good way to get the most out of your conversations is to come prepared with a list of questions. What would you like to discuss? What’s been on your mind since your last appointment? If you are looking for answers about something you read online, bring a copy of it to your appointment.
Here are some questions to help you get started with your own list:
- How have these treatment options been studied?
- What was the experience of others who received these treatments?
- Why do you think this treatment may be best for me?
- What can I expect from this treatment?
It can also be helpful to seek out additional information and support from communities of people who understand what you are going through. SHARE Cancer Support is a great place to start. This article was co-developed by Pfizer and SHARE Cancer Support.

SHARE is a national nonrofit that supports, educates, and empowers anyone who has been diagnosed with breast or gynecologic cancers, and provides outreach to the general public about signs and symptoms. We are a compassionate community of knowledgeable survivors, those living with cancer, and healthcare professionals.
SHARE is dedicated to serving people of all races and cultures, backgrounds, and identities. Because no one should have to face breast, ovarian, uterine, cervical, or metastatic breast cancer alone.
PP-UNP-USA-3731
© 2024 Pfizer, Inc. All rights reserved. March 2024
















